Lithium-ion Batteries
Air control Engineering Co., Ltd.
Lithium ion battery recycling
With the growth of the electric vehicle (EV) industry and rising global demand for rare metals, there is an increasing need for recycling processes that recover rare metals from used lithium-ion batteries. This trend has spurred expanded development and investment in these technologies. As an environmental technology company, we have a deep understanding of the recycling process for used lithium-ion batteries and possess advanced technology for effectively treating various hazardous chemicals and gases generated during this process. We are the only company in the country with expertise in the independent design, manufacturing, overseas installation, and operational data (OP DATA) of hazardous gas treatment systems.
Lithium-Ion Battery Recycling
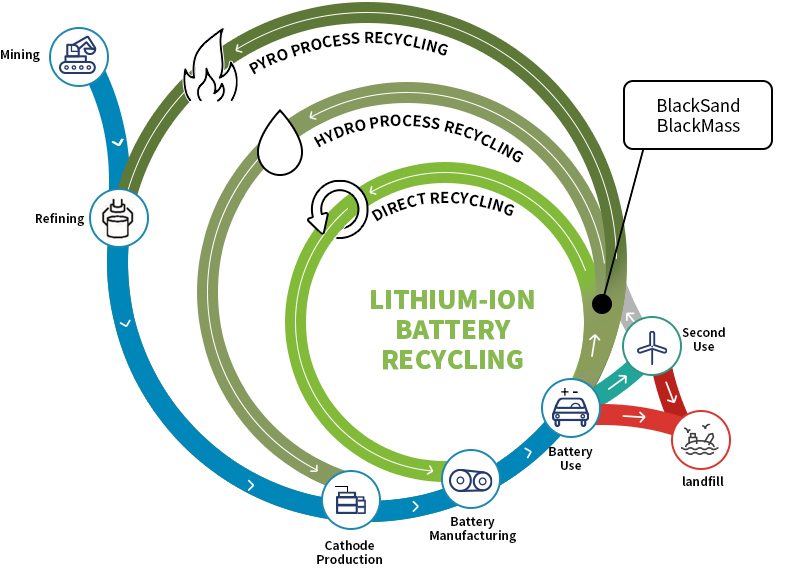
Battery Composition and Contaminants
Battery Composition
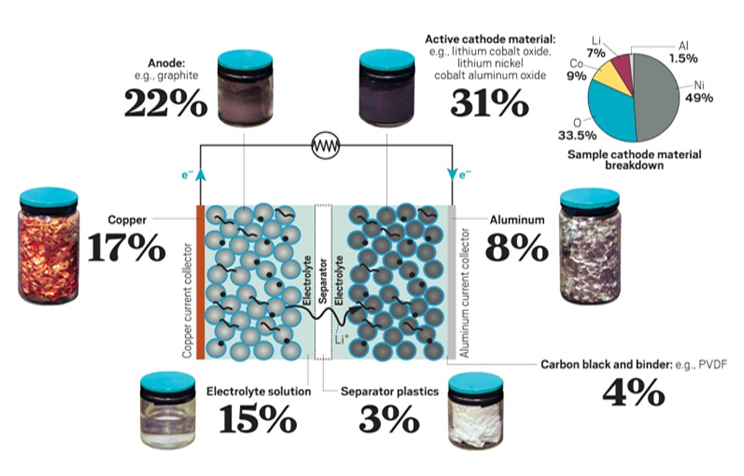
Effects of various components of lithium-ion batteries on the environment and human beings
| Material Types | Substances | Chemical Properties | Impacts |
|---|---|---|---|
| Cathode Material | LiCoO2, LiMnO4, LiFePO4, etc. | Reacts with acids and bases, producing heavy metals | Heavy metal contamination, acid rain |
| Anode Material | Graphite, etc. | Produces CO and particulate matter (DUST, FUME) during combustion | Air pollution from fine dust |
| Electrolyte Solute | LiPF6, LiBF4, LiCIO4, etc. | Reacts with water or generates strong corrosive/toxic gases upon exposure | Skin disorders, respiratory damage |
| Electrolyte Solvent | EC, EMC, DMC, PC, etc. | Generates VOCs and produces CO when burned | Air pollution from odors |
| Other metals | PVDF | Produces HF when thermally decomposed or reacts with strong acids, alkalis, and alkali metals | Skin and respiratory irritation |
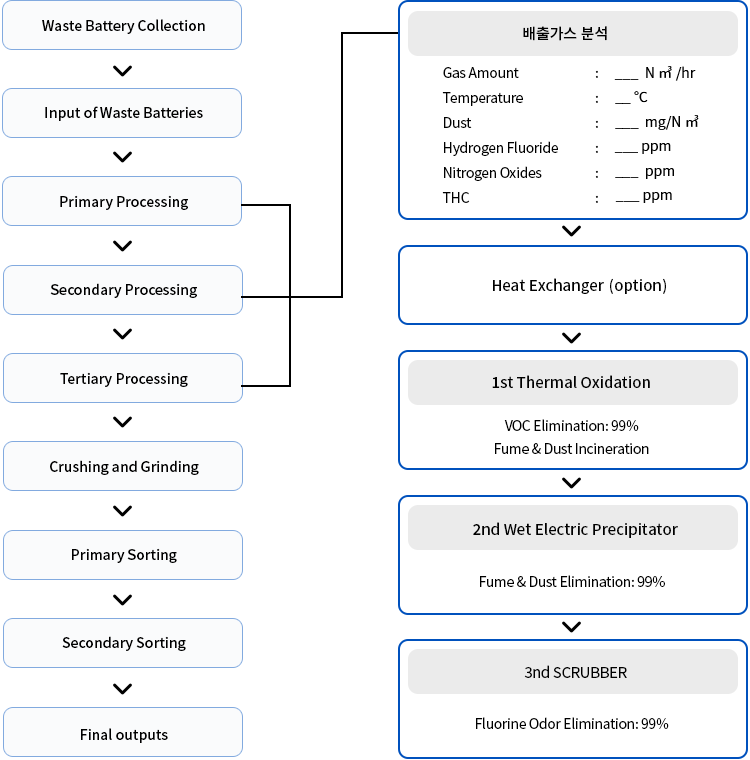
Treatment Flow
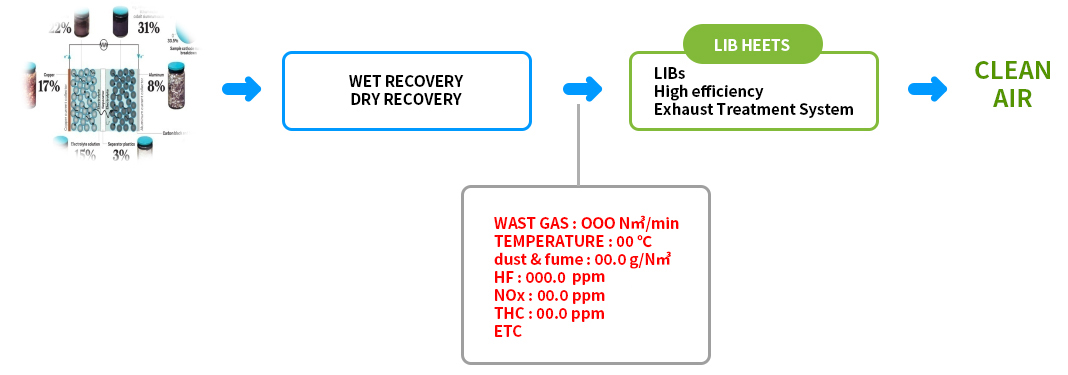
LIB HEETS
- 1 Organic compounds and VOCs are first thermally oxidized at high temperatures. The high-temperature energy released is then recycled through heat exchange or steam generation to save energy.
- 2 The thermally oxidized gases pass through a packing zone with KOH or NaOH for aqueous treatment. Remaining particulate contaminants (dust and fume) are then treated using a wet electrostatic precipitator.
- 3 Strongly acidic fluorine-based gases (F2, HF, BF3, CIF3) are neutralized with KOH solution in a scrubber for final treatment.
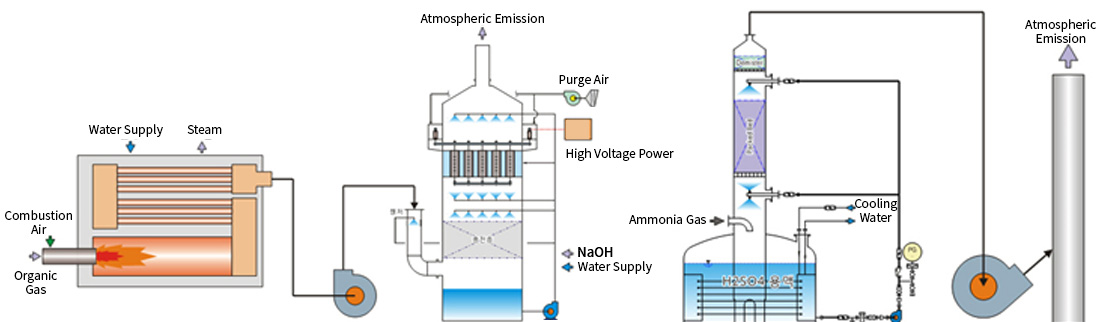
LIB Treatment Process Flowchart
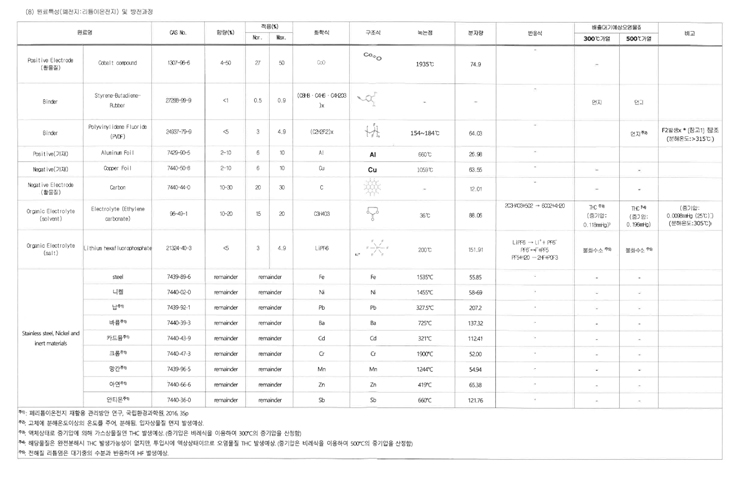
Battery Analysis
| Material Name | CAS No. | Concentration(%) | Application(%) | Chemical Formula | Structural Formula | Melting Point | Molecular Weight | ||
|---|---|---|---|---|---|---|---|---|---|
| Nor. | Max. | ||||||||
| Positive Electrode (Active Material) | Cobalt compound | 1307-96-6 | 4-50 | 27 | 50 | CoO | 1935℃ | 74.9 | |
| Binder | Styrene-Butadiene-Rubber | 27288-99-9 | <1 | 0.5 | 0.9 | (C8H8·C4H6·C4H2O3)x | - | - | |
| Binder | Polyvinylidene Fluoride (PVDF) | 24937-79-9 | <5 | 3 | 4.9 | (C2H2F2)x | 154~184℃ | 64.03 | |
| Positive(Conductive Material) | Aluminum Foil | 7429-90-5 | 2-10 | 6 | 10 | Al | 660℃ | 26.98 | |
| Negative(Conductive Material) | Copper Foil | 7440-50-8 | 2-10 | 6 | 10 | Cu | 1059℃ | 63.55 | |
| Negative Electrode(Active Material) | Carbon | 7440-44-0 | 10-30 | 20 | 30 | C | - | 12.01 | |
| Organic Electrolyte(solvent) | Electrolyte (Ethylene carbonate) | 96-49-1 | 10-20 | 15 | 20 | C3H4O3 | 36℃ | 88.06 | |
| Organic Electrolyte(salt) | Lithium hexafluorophosphate | 21324-40-3 | <5 | 3 | 4.9 | LiPF6 | 200℃ | 151.91 | |
| Stainless steel, Nickel and inert materials | steel | 7439-89-6 | remainder | remainder | Fe | Fe | 1535℃ | 55.85 | |
| Nickel | 7440-02-0 | remainder | remainder | Ni | Ni | 1455℃ | 58-69 | ||
| Lead Note)1 | 7439-92-1 | remainder | remainder | Pb | Pb | 327.5℃ | 207.2 | ||
| Barium Note)1 | 7440-39-3 | remainder | remainder | Ba | Ba | 725℃ | 137.32 | ||
| Cadmium Note)1 | 7440-43-9 | remainder | remainder | Cd | Cd | 321℃ | 112.41 | ||
| Chromium Note)1 | 7440-47-3 | remainder | remainder | Cr | Cr | 1900℃ | 52.00 | ||
| Manganese Note)1 | 7439-96-5 | remainder | remainder | Mn | Mn | 1244℃ | 54.94 | ||
| Zinc Note)1 | 7440-66-6 | remainder | remainder | Zn | Zn | 419℃ | 65.38 | ||
| Antimony Note)1 | 7440-36-0 | remainder | remainder | Sb | Sb | 660℃ | 121.76 | ||
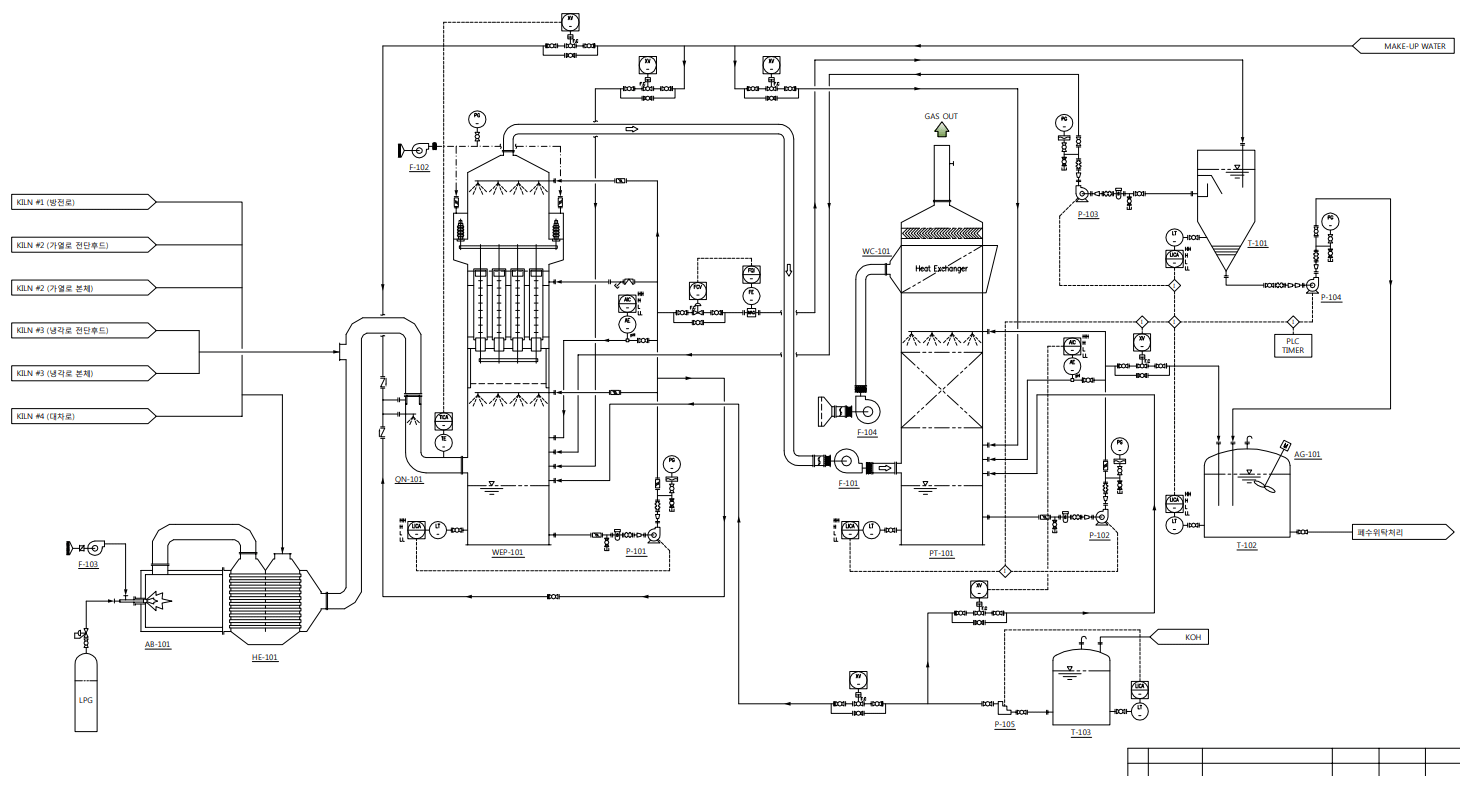
Construction Cases (P&ID)
Construction Cases (LAY OUT)
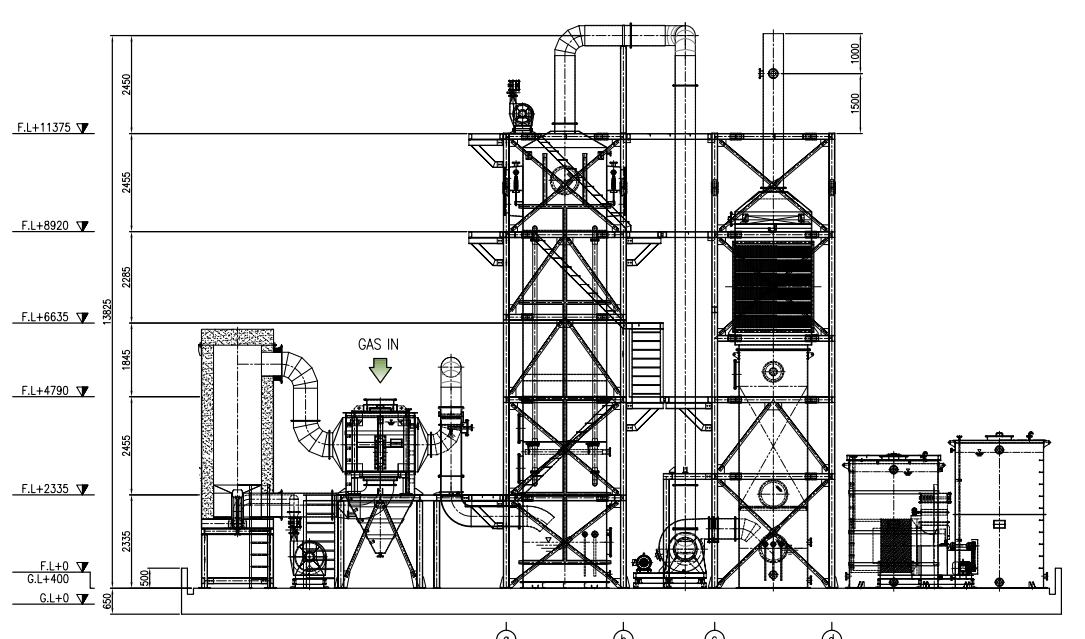
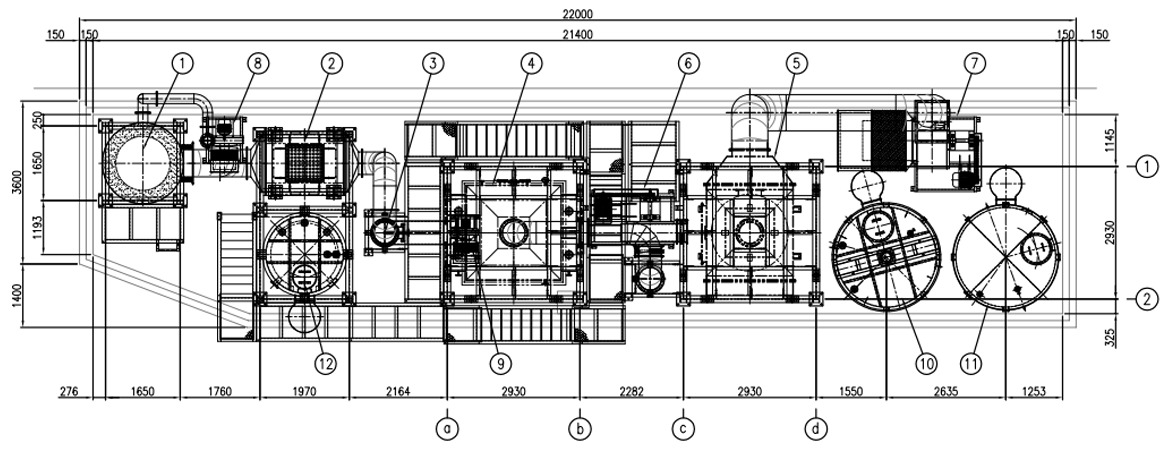
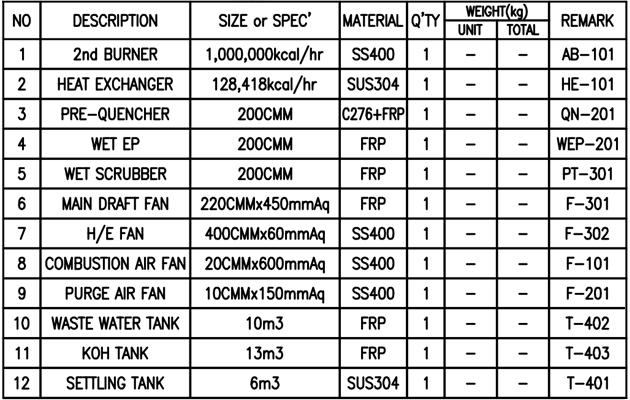
Construction Cases (HEETS LAY OUT)
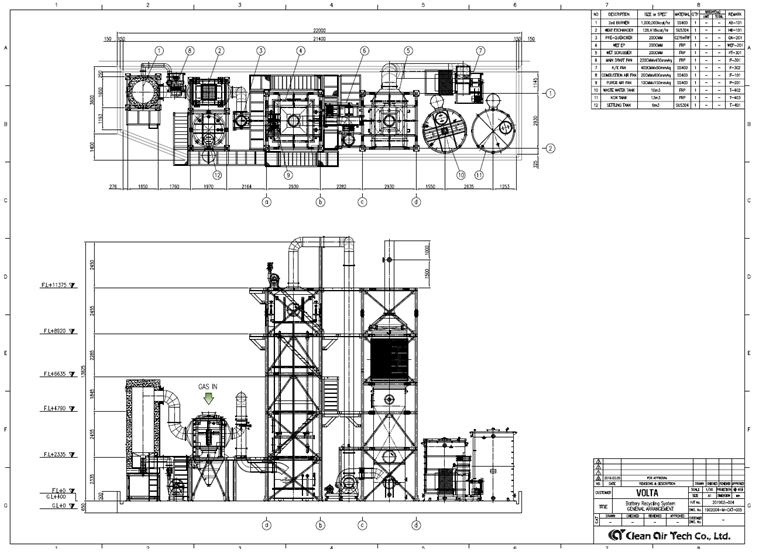
Construction Case (Photos)
Construction Case (Photos)
- ( Case 2 )

- ( Case 3 )



